Drawing-Up a SSCP (Summary of Safety and Clinical Performance)
Pitfalls & Solutions
General Background
The EU MDR comprises a couple of novelties and obligations for manufacturers to be fulfilled versus the MDD. Among others, the Summary of Safety and Clinical Performance (SSCP) is an important document to be drawn up by manufacturers for implantable and class III medical devices according to Article 32 EU MDR. The MDCG 2019-9 Rev. 1 guidance document covers details about the content, process of preparation and upload of the SSCPs in EUDAMED, including a template.
The SSCP is a publicly available document, which will be stored in EUDAMED, once the database is fully functional. The name of the document already points on its content, which is outlined below.
Required sections of the SSCP document
- The identification of the device and the manufacturer, including the Basic UDI-DI and, if already issued, the SRN
- The intended purpose of the device and any indications, contraindications and target populations
- A description of the device, including a reference to previous generation(s) or variants if such exist, and a description of the differences, as well as, where relevant, a description of any accessories, other devices and products, which are intended to be used in combination with the device
- Information on any residual risks and any undesirable effects, warnings and precautions
- The summary of clinical evaluation as referred to in Annex XIV, and relevant information on post-market clinical follow-up
- Possible diagnostic or therapeutic alternatives
- Suggested profile and training for users
- Reference to any harmonised standards and CS applied
- Revision history
When combining the aforementioned information about required content and intended target group(s), specific purposes of a SSCP may become obvious (below, left site). On the contrary a SSCP is not to be regarded a substitute for other documents are even professional healthcare consultancy as outlined below, right site.
What is the rational/purpose of the SSCPs for MDs?
Purpose
It should …
- Provide public access for the target groups with current & updated information about the device’s
- Intended purpose, function, etc.
- Safety aspects of the device
- FSCA, FSN
- Clinical Performance of the device
- Clinical benefits for patients
- Target the following (user) groups
- HC-professionals
- Patients as indirect end-user or direct user (if applicable)
- Finally enhance transparency and provide adequate access to information
Not the Purpose
It should not …
- Give general advice on the diagnosis or treatment (therapeutic suggestions) of particular medical conditions for users or patients, nor
- Replace the IFU as the main document that will be provided to ensure the safe use of a particular device, nor
- Replace the mandatory information on implant cards or in any other mandatory documents , nor
- Replace consultation with a HC professional if needed, nor
- Be used/understood as marketing or sales leaflet!
Distinct aspects of a SSCP
As the SSCP is an integral part of the conformity assessment process, it is suggestive to take a closer look on the requirements before drawing up the document.
The following important aspects may be considered:
- The SSCP is an integral part of the TD (Technical Documentation)
- The SSCP needs to be validated by the notified body, which applies for the initial certification and all updates
- The target group of the SSCP are healthcare professionals and/or – in certain cases – patients
- Besides content, a couple of stylistic requirements apply for drawing up compliant SSCP's, specifically regarding the part which is for patients
- The SSCP is required to be kept up-to-date
“Feeding” the SSCP with content
For drawing up a SSCP, it is not required to retrieve or produce new data. The SSCP should be fully sourced from Documents of the TD, outlined below. Thus, it may become obvious that all source documents should be in good shape before drawing up the SSCP document. It makes no sense, to start drawing up a SSCP before the CER, IFU and other documents of the TD are finalized.
The SSCP is a the end of the food chain …
The SSCP should be fully sourced from the TD according to Annex II and Annex III of the EU MDR.
Source documents mainly comprise:
- Clinical Evaluation Report (CER)
- Instructions for Use (IFU)
- Risk-Management Documents (RM)
- Post-Market Surveillance documents (PMS/PMCF)
- Other objective evidences from the TD (e.g. design verification/ validation reports – providing e.g on info about previous generations / differences, reasons for changes …)
If you cannot easily feed the SSCP from the these docs, consider that the CER or other documents are not in good shape!
Revise them first - prior to finalizing the SSCP.
When is a patient part required?
A patient part of a SSCP is required for (a) implantable devices for which patients will be given implant cards and (b) Class III devices that are intended to be used directly by patients (Note: Devices listed in MDR Annex XVI, and eligible for a SSCP, should always be considered as relevant for patient information).
Stylistic and language Requirements
To make sure, that the patient’s part of the SSCP is understood by laypersons, the MDCG guidance requires using lay language. To verify readability – or better understandability of the content – it is suggestive to use a combination of readability tests (e.g. Flesch-Reading-Ease-Score) and surveys, carried out with a representative target population. Nonconformities are often raised by notified bodies, because the language used for the patient part is not comprehensible for laypersons.
During the required validation step, the assessment of a draft SSCP will be carried out by the notified body in one defined language. The translation into all other languages is in the manufacturers ‘responsibility. Further recommendations are outlined below.
Requirements to a SSCP / Expectations of the NB?
- Technically: Printable / searchable format (PDF)
- Layout: Font type and size should allow easy reading, well structured/organized (using e.g. headlines, paragraphs)
- Content: Easy to read, concise, unequivocal & clear, objective, adequately summarising both favourable and unfavourable data
- Language: Comprehensible to patients and lay persons (for the patient part)
- The SSCP is not meant to be a marketing or sales leaflet. See MDCG 2020-19 Rev 1.

The SSCP in the landscape of the Technical Documentation
The SSCP is closely related to a couple of other documents from the TD as outlined in the figure below. It is of utmost importance, to have the content of the SSCP aligned with the corresponding documents of the technical documentation.
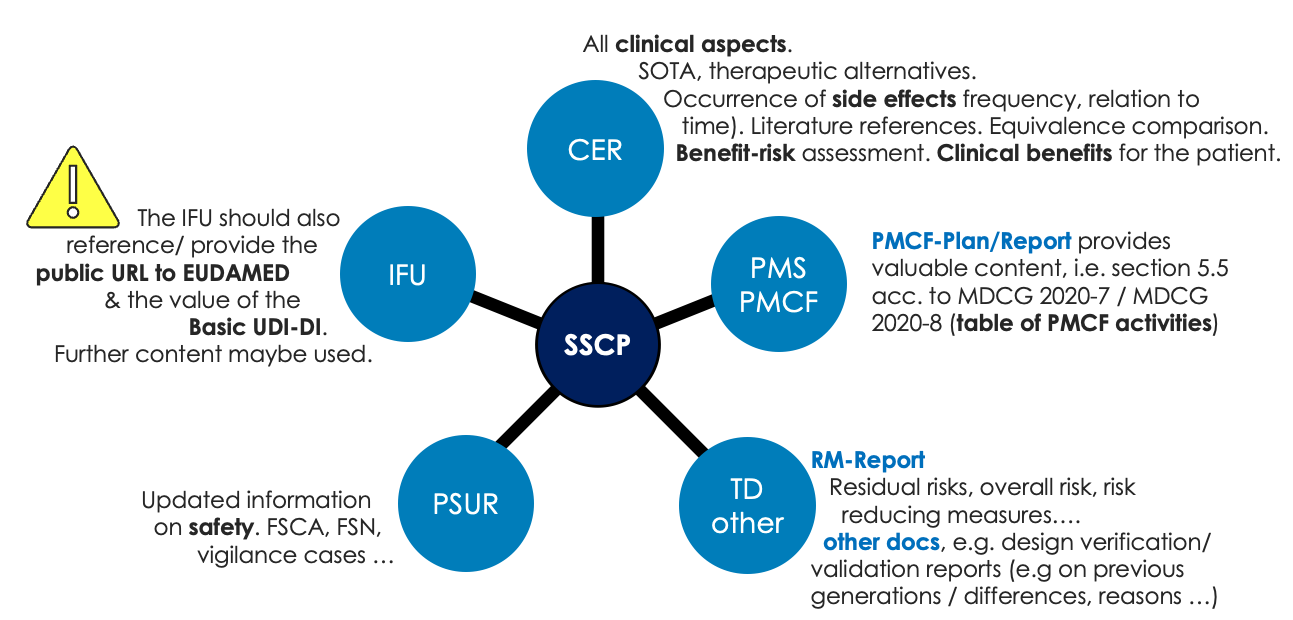
The SSCP contains 2 important abbreviations: S = Safety and CP = Clinical performance. As such, the SSCP is very closely connected to the RMF (Risk Management File), CER (Clinical Evaluation Report), PMCF (Post Market Clinical Follow-Up) and PSUR (Periodic Safety Update Report).
Whenever one of these documents will be updated during the lifetime of the device, the SSCP needs to be aligned accordingly. Note: Updates might require involvement of the notified body, depending on the risk class of the device. In turn, update the SSCP at least with the annually updates of the PSUR and PMCF, respectively (if necessary).
Pitfalls and Solutions
Although the MDCG 2019-9 Rev 1 contains a template and a detailed description of the requirement, certain sections are prone to findings by the notified body. These are specifically the section 4 (Risks and warnings > Quantifications and relation of time) and section 5 (Summary of clinical evaluation and post-market clinical follow-up > The overall summary of the clinical performance and safety versus state-of-the-art). For a successful verification by the notified body it is important to carefully follow the instructions as outlined in the MDCG 2019-9 Rev 1 guidance.
If you need support, AKRA TEAM would be more than happy to support you!
Support & Training
Contact AKRA TEAM for support, hands on implementation services and personalized training by experts with key competencies in the areas listed below.
Key points
The SSCP is an important document during conformity assessment procedure. Although a MDCG guidance is available for drawing up SSCPs, the description in MDCG 2019-9 Rev 1 appears to be equivocal in some areas, thus leading to different interpretation by manufacturers and therefore to varying output. This – in turn – might impede assessment by notified bodies or leading even to rejection of the document.
To put it into a nutshell: It is important to pay meticulous attention when drawing up SSCPs and consider a couple of topics, as summarized below.
SSCP: What can I do to comply with the legislation?
IMPORTANT TO NOTE
There are different templates for healthcare professionals and patients, respectively. Carefully check, whether the medical device is to be used by the patient directly and if implant cards apply for the device. In both cases, a patient part is mandatory. MDCG 2019-9 Rev. 1 recommends upfront statements for healthcare professionals and patients. Include these passages in the SSCP.
Some suggestions. Make sure …
that your sourcing documents are in good shape
to follow the numbering and structure, proposed in the templates (see MDCG 2019-9 Rev. 1)
that the output files are searchable and printable (PDF)
to reference the SSCP in the IFU
using “lay language” for the patients part and avoid medical terms
to explain all abbreviations or use a List of Abbreviations
to align content with the CER, IFU etc.
to exclude elements of promotional nature
to present the content in an organized and unambiguous manner
to use the EMDN codes for the devices
Our Services
Consultancy prior to draw up a SSCP document by our experts.
Gap-Assessment / readiness check of SSCP drafts including detailed recommendations for improvements.
Consultancy on Non-Conformities / Deficiencies raised by the notified body. Support on response letters to the notified body.
MDR compliant SSCP template with comprehensive descriptions. This will help you drawing up the SSCP and considering the required aspects.
Implementation of the SSCP process as part of the certification process.
Webinar/Training on drawing up SSCPs (Basics / Pitfalls & Solutions / Recommendations).
Implementation of the SSCP process as part of the certification process.